“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,” Doctor Nicole Verdun, director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research, said in a statement. “Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited.”
The life-long genetic condition is caused by errors in the genes for the protein hemoglobin. Red blood cells use hemoglobin to carry oxygen through the body. The abnormal hemoglobin makes the blood cells crescent-shaped and hard. The misshapen cells then clump together and block the flow of oxygen to the organs, which causes extreme pain. The cells can then die off early, which leads to anemia.
Sickle cell disease is particularly common among people with Caribbean or African ancestry and affects more than 100,000 Americans. Previously, the only known cure for sickle cell disease was a bone marrow transplant from a donor. These procedures come with the risk of rejection by the patient’s immune system and finding a matching donor is a difficult process.
How Casgevy works
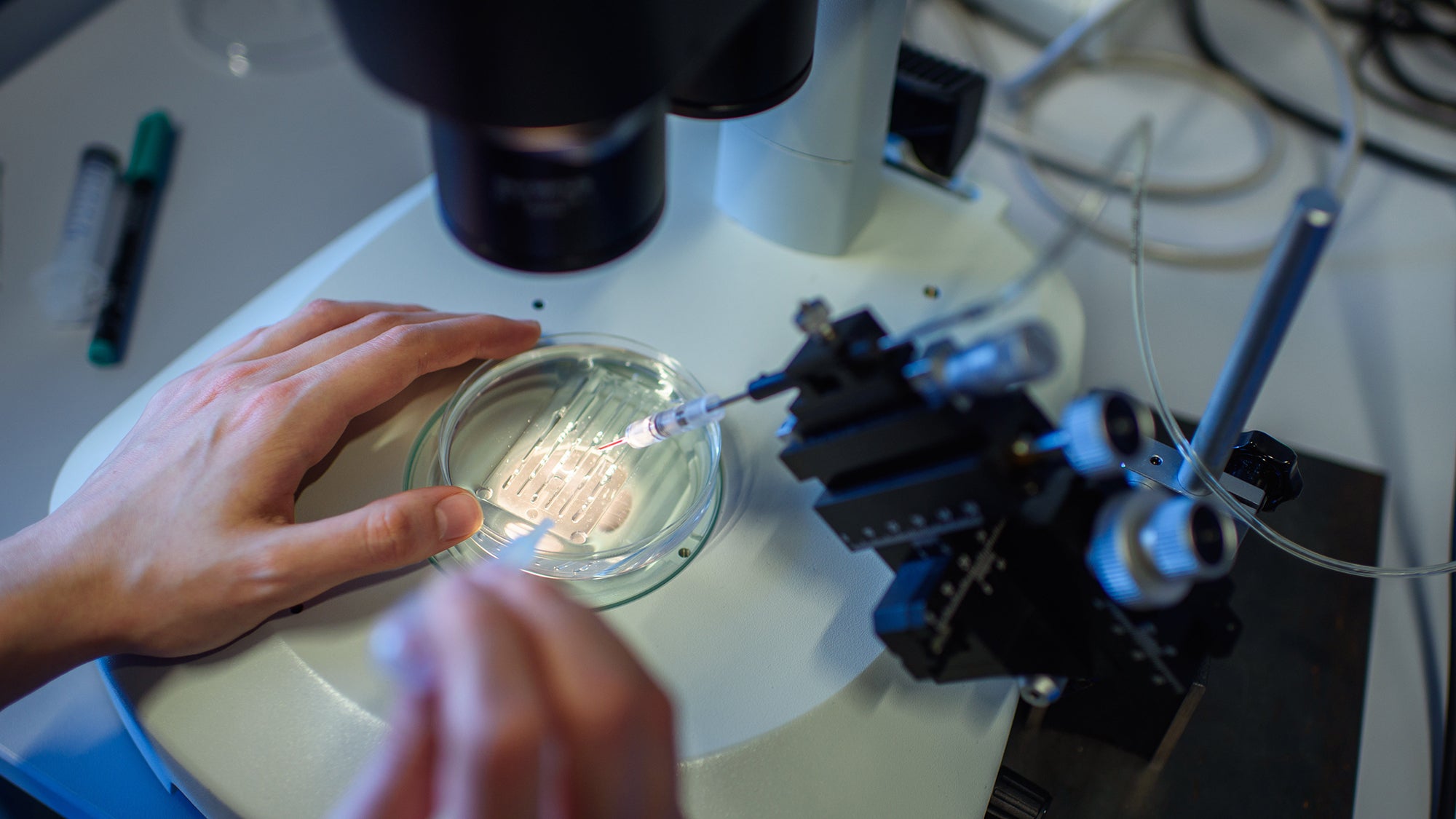
Casgevy uses CRISPR-Cas9 gene editing technique, which allows scientists to make precise alterations to human DNA. French microbiologist, geneticist, and biochemist Emmanuelle Charpentier and American biochemist Jennifer A. Doudna shared the 2020 Nobel Prize in Chemistry for their work developing the technique.
The procedure uses stem cells taken from a patient’s bone marrow. The cells are then brought into a lab and the genes that are meant to switch on a functioning version of hemoglobin are edited with CRISPR. Patients must then go through a “conditioning treatment.” This can involve taking a drug that suppresses the immune system, radiotherapy, or chemotherapy to get the body ready for an infusion of CRISPR-modified cells back int…